Business License (Company Registration)

View LAEYO Labs’ business license and company registration details for supplier verification, including registered entity information and audit-ready documentation context.

Call(WhatsApp Us)
+8618988990383Email Us
postmaster@laeyolabs.com广州市花都区花山镇华辉路18号
微观化妆品创意产业园E栋整栋Evidence-first manufacturing for due diligence: certifications, compliance documents, factory & process proof, patents, and test reports. Request the exact audit pack for your market
Each evidence card shows a clear title, a preview image, and a short intro so buyers can scan the library quickly. Need the full audit pack for a specific SKU or market? Request the exact set and we will share the appropriate files.
We do not publish exaggerated testimonials or unverified claims as structured data. Case studies and customer references are shared only with approval or under NDA.
Use this library to speed up supplier due diligence. Each report includes a clear title, a preview, and a short summary—request the full files for your SKU and target market.
View LAEYO company credentials, including business registration, manufacturing/production permits, institute qualifications, and operating licenses—organized for supplier verification and audits.

View LAEYO Labs’ business license and company registration details for supplier verification, including registered entity information and audit-ready documentation context.

Zhongke Skin Gene (Guangdong) Institute of Dermatological Research is a dermatology-focused research institute engaged in skin science, ingredient efficacy evaluation, and applied research to support evidence-based skincare product development.

Official cosmetics manufacturing license for LAEYO Labs, issued by the competent authority. Covers permitted product scope and manufacturing activities, with validity details and audit-ready documentation for supplier verification.

National Enterprise Credit Information Publicity System is the official Chinese government database for verifying company registration, legal status, shareholders, and compliance disclosures. It is used for supplier due diligence and entity verification.

This license authorizes LAEYO Labs’ China entity to operate approved value-added telecommunications services in accordance with PRC regulations. It applies to online information and digital service operations

Zhongke Microview (Guangdong) Medical Research Institute conducts applied research in medical and life sciences, supporting formulation research, technical validation, and scientific assessment for industry partners.
Use this Compliance Documents library to speed up supplier due diligence and reduce market-entry risk. Each card includes a clear title, a redacted preview, and a short summary covering common buyer requirements—SDS/MSDS, COA, INCI ingredient listings, IFRA (for fragrance), declarations, and shipping classification. Request the full compliance pack for your specific SKU and target market, and we’ll share the appropriate files (NDA-supported when needed) and typically reply within 8 hours.
 PDF
PDF
Third-party hazard identification report confirming the listed product can be transported as non-dangerous goods, with itemized checks covering explosive risk, flammability, oxidation/organic peroxide, toxicity & infection, radiation, corrosion, and other transport hazards—supporting safer, compliant shipping documentation.
 PDF
PDF
Redacted MSDS/SDS documenting product safety and handling controls, including hazard identification, composition overview, first-aid and fire-fighting guidance, storage/handling requirements, exposure controls, disposal guidance, and transport classification.
 PDF
PDF
This Sea Freight Identification & Classification Report (DGM China, “By Sea”) assesses the listed cosmetic goods against IMDG Code criteria and confirms they are “Not subject to these regulations” for sea transport, with hazard-screening results covering flammability, oxidizing properties, toxicity/infection, corrosion, and other risks
Factory & Process Proof is your visual due-diligence library for cosmetic OEM/ODM and private label production at LAEYO—clean production routines, batching and emulsification, filling, packaging, and final packout. These short, unedited clips help you verify GMP-aligned controls (ISO 22716-style workflows), hygiene discipline, in-process QC checkpoints, and batch traceability before you request samples or submit an RFQ. If you need an audit pack for a specific SKU and target market, tell us your requirements and we will share the relevant SOP excerpts and supporting records.

A close-up of our lab emulsification step—high-shear mixing to build a stable, uniform texture before moving into evaluation and scale-up planning.

A look at our compounding area with stainless-steel mixing vessels and control panels—built for controlled processing and consistent batch production.

Automated pouch feeding and conveying on the packaging line—organized material flow designed to improve consistency, efficiency, and in-process handling control.

Inside our formulation lab: raw materials are staged and weighed on calibrated scales, then prepared in controlled steps to support repeatable prototypes and traceable development.

Unedited bench-side footage showing gift set components being placed into a custom foam insert for consistent presentation and packout—prior to overwrap/cartoning and shipment.

A quick look inside our controlled production room: operators in protective gear assemble components on a moving line with step-by-step handling and in-process checks to keep output consistent and contamination risk low.

Unedited on-site footage showing finished cartons being placed into a clear protective overwrap and sealed at the packing station—helping keep boxes clean and protected before outer-case packing and shipment.

Unedited footage from our packaging area: operators assemble and pack retail cartons on a conveyor line, supporting consistent packout and pre-shipment checks before outbound QC.

A real-time look at our emulsification step: controlled stirring and mixing to achieve uniform texture and batch consistency before filling and packaging.

A short, unedited look at our controlled packaging area: operators assemble cartons on a conveyor line under hygiene controls to support consistent packout and pre-shipment checks.

A short, unedited view of automated cartoning. This step helps keep packout consistent and reduces handling variability before outbound QC and shipment.

A quick look inside our controlled production area: stainless mixing/emulsifying tanks and operator-controlled processing.
Use this Patents & IP library to support supplier onboarding and audit prep. Each card lists the patent title, registration number, and a concise summary—so you can screen relevance fast, then request the full files if needed.

repair composition and its preparation method and its applications

hydrating moisturizing composition and its preparation method and its applications

filling device for a ace mask filling machine

anti sensitivity repair composition and its preparation method and its applications

a vacuum drying oven for cosmetics

a rotational viscometer for laboratory use

a pure water system with sterilization and disinfection functionality

a mixing equipment for cosmetics production

a homogenizer for cosmetics laboratory use

a high pressure autoclave for cosmetic ingredient -production

a filling machine-to improve filling stability

a biochemical incubator for cosmetics
Each functional product is supported by independent third-party testing. Below is a curated selection of reports that document key quality, safety, and performance indicators (for example, microbiological limits and regulated contaminants where applicable).
 PDF
PDF
Independent lab report for “CH Proxylane Firming Cream” verifying key safety compliance items under the Cosmetic Safety Technical Specification, including microbiological limits (total count, yeast & mold, thermotolerant coliforms, S. aureus, P. aeruginosa) and chemical/contaminant limits (Hg, Pb, As, Cd, and 1,4-dioxane). Results are documented as meeting the applicable requirements for an audit-ready quality evidence […]
 PDF
PDF
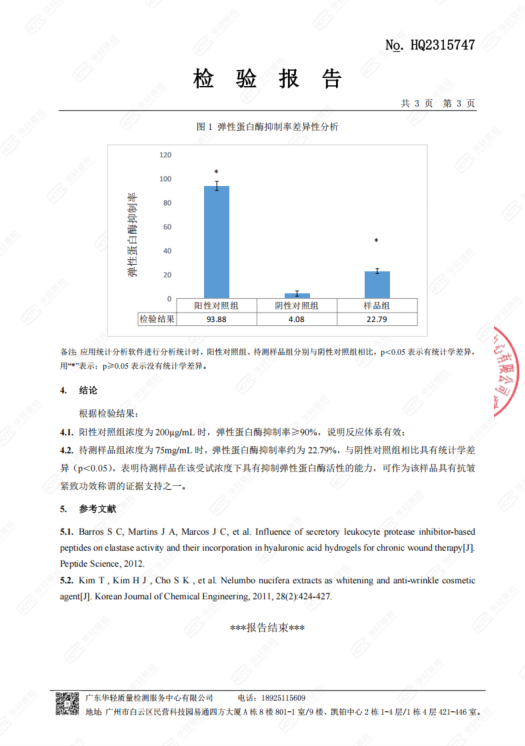
Independent lab test report commissioned by LAEYO for “CH Proxylane Firming Cream”, evaluating elastase inhibition as an in-vitro indicator for anti-wrinkle/firming performance. The report provides the test method, quantified results, statistical analysis, and conclusion to support claim substantiation in an audit-ready evidence pack.
 PDF
PDF
Third-party cosmetic efficacy report documenting the test design, methodology, data charts, and conclusions for a Proxylane 30% sample, supporting repair-claim substantiation as part of our formulation validation and evidence pack
Empowering your brand with our top OEM/ODM cosmetic solutions